Introduction: The Dawn of AI-Powered Molecular Discovery
The relentless march of artificial intelligence continues to redefine the boundaries of human knowledge and capability. Across an ever-expanding array of disciplines, AI is emerging as a powerful catalyst for innovation, and nowhere is this more profoundly felt than in the complex and vital realms of biology and medicine. At the forefront of this scientific revolution is the unveiling of the **AlphaFold 3 AI model for drug discovery**, a groundbreaking advancement poised to fundamentally alter our comprehension of diseases and the development of novel therapeutic strategies.

Understanding the intricate dance of protein folding is central to unlocking the secrets of biological processes. Proteins, the workhorses of our cells, must fold into precise three-dimensional structures to perform their myriad functions. Errors in this delicate folding process are intrinsically linked to a vast spectrum of diseases, from neurodegenerative disorders like Alzheimer’s to metabolic conditions and cancers. Consequently, unraveling these folding mechanisms and the resulting protein structures is paramount for the development of effective treatments.
AlphaFold 3 arrives not merely as an incremental upgrade, but as a paradigm shift. Its capabilities promise to dramatically accelerate the often lengthy and arduous drug discovery pipeline, offering unprecedented speed and accuracy in predicting molecular structures and their interactions. This heralds a new era where the power of AI is directly harnessed to tackle some of humanity’s most pressing health challenges.
The Genesis of a Revolution: AlphaFold’s Legacy in Protein Folding
The journey towards AlphaFold 3 is built upon a foundation of significant scientific achievement. The initial release of the **Google DeepMind protein folding breakthrough** marked a pivotal moment in computational biology. For decades, predicting how a linear chain of amino acids folds into a functional three-dimensional protein structure was one of science’s most formidable challenges. The sheer number of possible configurations a protein sequence can adopt is astronomically large, making brute-force computation an impractical endeavor. This complex puzzle was finally addressed with groundbreaking accuracy thanks to the AI models developed by DeepMind [source: prescouter.com, blog.google].

_Protein folding_, in essence, is the physical process by which a polypeptide chain, the linear sequence of amino acids, spontaneously coils and arranges itself into a specific, stable, and biologically active three-dimensional shape. This precise architecture is what dictates a protein’s function, whether it’s acting as an enzyme to catalyze reactions, a structural component, a signaling molecule, or a transporter. Any deviation from this native state, often caused by genetic mutations or environmental factors, can lead to misfolding, aggregation, and the onset of disease.
Earlier iterations of AlphaFold, most notably AlphaFold 2, represented a monumental leap forward. These models demonstrated an unprecedented ability to predict the structure of individual proteins with accuracy that rivaled experimental methods, often achieving near-atomic resolution. This disrupted traditional paradigms in structural biology, which relied on time-consuming and resource-intensive techniques like X-ray crystallography and cryo-electron microscopy. AlphaFold 2 democratized structural biology, making accurate protein structure predictions widely accessible and significantly accelerating research in countless areas of biological science [source: prescouter.com, blog.google].
Introducing AlphaFold 3: A New Era Under Isomorphic Labs
The baton of innovation has now been passed to **Isomorphic Labs**, a distinct entity spun out from Google’s DeepMind. Isomorphic Labs has unveiled its latest creation, **AlphaFold 3**, representing a significant generational leap forward in AI-driven molecular modeling. This new model directly builds upon the foundational successes and architectural insights gleaned from DeepMind’s pioneering work, pushing the boundaries of what’s possible in understanding molecular interactions [source: blog.google, nature.com].

The key advancements in AlphaFold 3 are truly remarkable. While its predecessors excelled at predicting the structures of individual proteins, AlphaFold 3 goes much further. It delivers atomic-level accuracy not only for single proteins but also for complex assemblies involving multiple components: proteins, nucleic acids (such as DNA and RNA), and even small molecules, including drug ligands. Crucially, it can also accurately model chemical modifications to proteins, which are vital for understanding their function, regulation, and involvement in disease states [source: labiotech.eu, prescouter.com, blog.google].

Underpinning these capabilities is a sophisticated technological foundation. AlphaFold 3 employs advanced deep learning architectures, similar to those used in cutting-edge image generation models, combined with a novel diffusion-based modeling technique. This approach allows it to construct highly accurate, joint 3D structures of entire molecular assemblies in a single, integrated process. The result is a predictive power that approaches, and in many cases surpasses, experimental methods in terms of speed and accessibility, offering near-experimental accuracy for these complex biological systems [source: blog.google].
The Broadening Scope: AI Advancements in Structural Biology with AlphaFold 3
AlphaFold 3 signifies a monumental step forward in the ongoing wave of **AI advancements in structural biology**. Its enhanced capabilities extend far beyond the prediction of isolated protein structures, offering a holistic view of the molecular machinery within cells. This represents a crucial evolution in how scientists can approach complex biological questions.

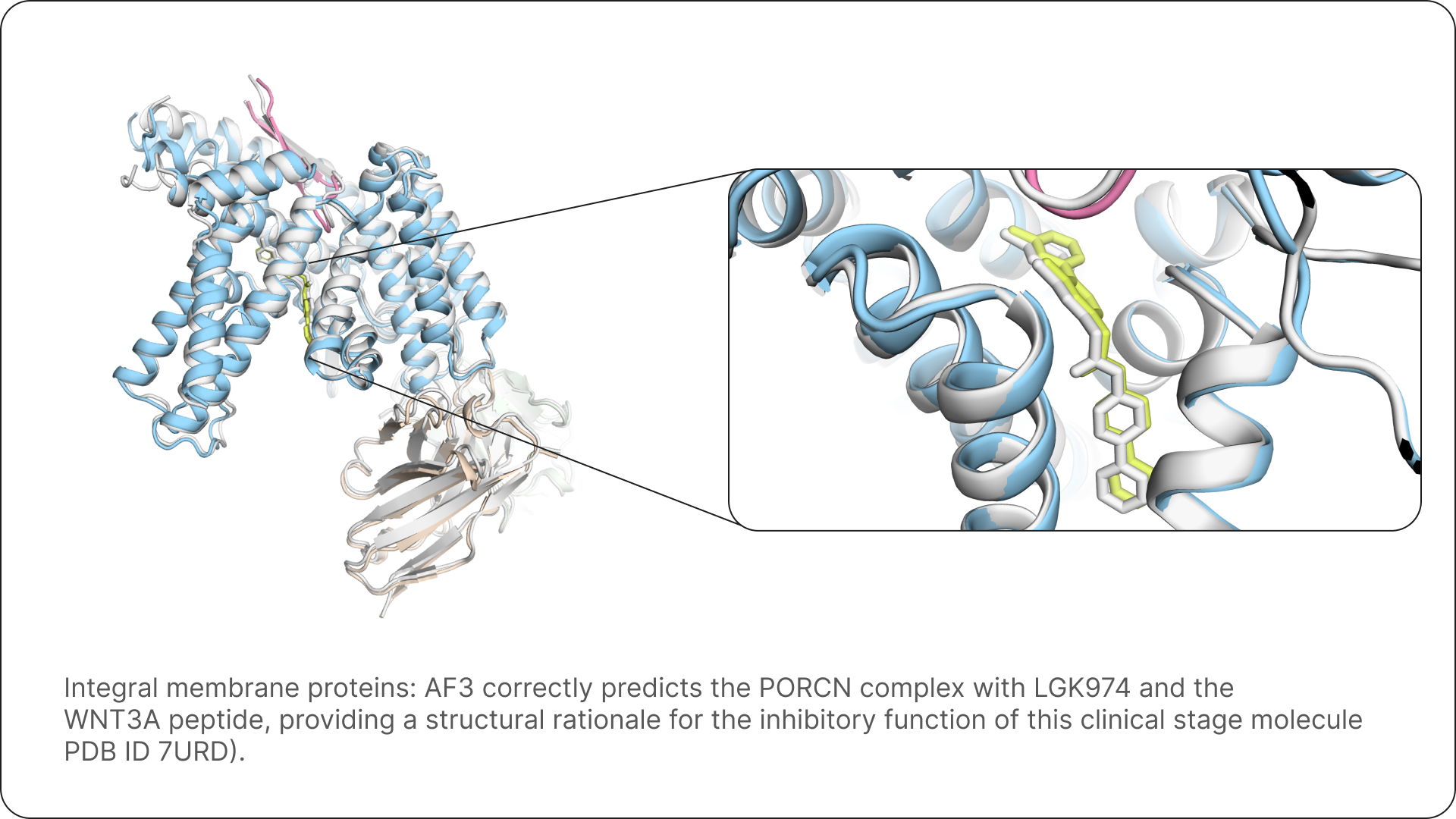
Unlike its predecessors, which primarily focused on single protein structures, AlphaFold 3 is adept at modeling a much wider array of intricate biological interactions. This includes predicting the structures of protein-protein complexes, which are fundamental to cellular signaling and function. Furthermore, it can accurately model how proteins interact with other crucial biomolecules, such as nucleic acids (DNA and RNA) and small molecules like drug candidates or metabolites. This broader scope allows for a more comprehensive understanding of biological pathways and disease mechanisms [source: labiotech.eu, prescouter.com, blog.google].
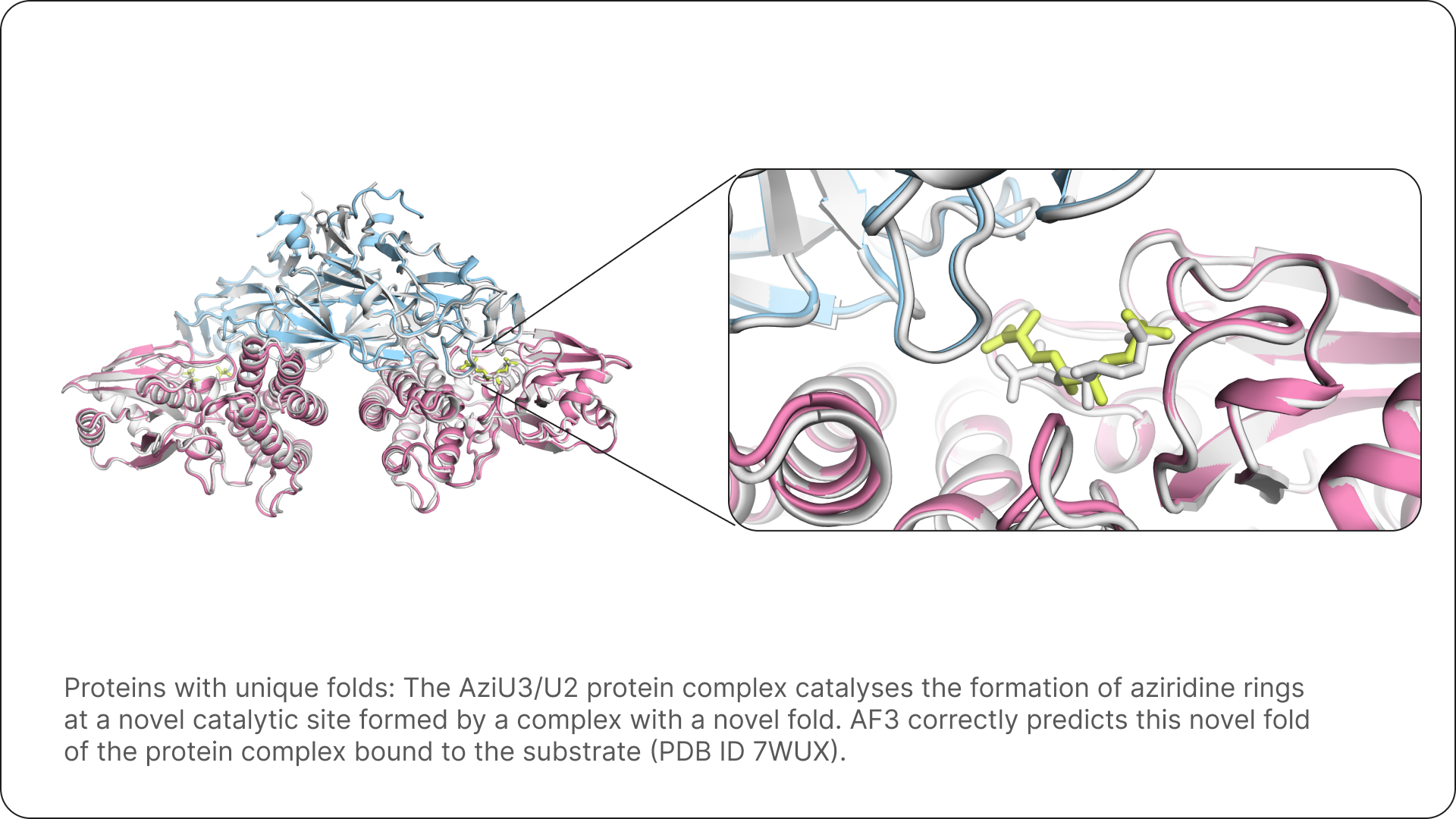
The platform’s holistic approach is a game-changer. By modeling entire molecular complexes in a single, integrated pass, AlphaFold 3 empowers scientists to investigate disease mechanisms and cellular functions with an unprecedented level of detail and reliability. This integrated view can often provide insights that are difficult or impossible to obtain through traditional, fragmented approaches that rely on separate experimental techniques or computational methods for each component. It allows for a more unified and accurate representation of how biological systems operate at the molecular level [source: labiotech.eu, prescouter.com, blog.google].
The performance gains are substantial. On rigorous benchmarks like PoseBusters, which evaluates the accuracy of predicted protein complex structures, AlphaFold 3 has demonstrated an impressive **50% improvement in accuracy** compared to existing state-of-the-art methods. This level of enhancement signifies a profound impact on the reliability and utility of computational predictions in structural biology research [source: labiotech.eu].
Transforming Medicine: The Impact of AlphaFold 3 on Drug Discovery
The true promise of AlphaFold 3 lies in its profound **impact on medicine**, particularly its transformative potential within the highly complex and resource-intensive field of drug discovery. The model’s ability to predict molecular structures and interactions with remarkable accuracy and speed has the potential to revolutionize how we develop new therapies.

AlphaFold 3 has the capacity to accelerate the entire drug discovery pipeline, from initial target identification through to lead optimization and even preclinical testing. Its predictive power can significantly reduce the time and cost associated with traditional methods, making the development of new medicines more efficient and accessible [source: labiotech.eu, prescouter.com, blog.google].

One of its most significant contributions is in the realm of identifying new drug targets. By accurately revealing how proteins interact with other biomolecules that are crucial to disease pathways, AlphaFold 3 provides researchers with a deeper understanding of disease mechanisms. This, in turn, expands the possibilities for identifying and validating novel molecular targets for therapeutic intervention. Precisely understanding the target means better drug design.

Furthermore, the platform offers immense utility in the drug design process itself. It can assist in optimizing the structure of lead compounds to enhance their efficacy and reduce potential side effects. AlphaFold 3 can also predict potential off-target interactions or toxicity, providing crucial insights into a drug candidate’s safety profile. This capability can significantly reduce the need for costly, time-consuming, and often failure-prone laboratory experiments early in the development cycle, allowing researchers to focus on the most promising candidates [source: prescouter.com].
The impact is already being felt within the pharmaceutical industry. Leading global pharmaceutical companies, such as Novartis and Eli Lilly, are actively forging collaborations with Isomorphic Labs. These partnerships are focused on integrating AlphaFold 3’s capabilities directly into their extensive research and development processes, signaling a strong industry-wide recognition of its transformative potential [source: nature.com].
.jpg)
The integration of AlphaFold 3 into these R&D pipelines promises to accelerate the development of more effective and safer drugs. This, in turn, could lead to the creation of novel treatments for a wide spectrum of diseases that currently have limited or no satisfactory therapeutic options, offering new hope to millions of patients worldwide [source: labiotech.eu, prescouter.com, blog.google].
Navigating the Horizon: Challenges and Future Prospects
While the capabilities of AlphaFold 3 are undeniably impressive, it’s important to acknowledge the existing challenges and to look towards the future prospects of AI in structural biology. The landscape of scientific research is complex, and the development and application of powerful AI models like AlphaFold 3 are not without their hurdles.

One notable challenge lies in the availability of comprehensive structural data, particularly for drug-bound protein complexes. Much of this data remains proprietary, held within private companies, which can limit open research and the further development and refinement of AI models. Moreover, accurately modeling certain complex molecular interactions, especially those involving transient states or less well-characterized biological systems, remains an area of active research and development. The nuances of biological systems mean that while AI provides remarkable accuracy, it is an evolving field that constantly seeks to improve its predictive power across all scenarios [source: nature.com].

Looking ahead, the trend is clearly towards greater integration of larger, more diverse datasets into AI training. Fostering collaboration among leading AI researchers, pharmaceutical companies, and public research institutions will be crucial for advancing the field collectively. Open-source initiatives and data-sharing platforms are vital for democratizing access to these powerful tools and accelerating the pace of discovery for the entire scientific community [source: nature.com].

Ultimately, AlphaFold 3 serves as a compelling example of the future of AI-driven medicine. It demonstrates a profound capability to accelerate basic scientific discovery, revolutionize the methodologies of drug design, and offer new avenues for precision-based disease treatment that were once considered unattainable. The synergy between AI and scientific innovation is forging a path toward unprecedented progress in healthcare [source: labiotech.eu, prescouter.com, nature.com].
Final Thought
The **AlphaFold 3 AI model for drug discovery** represents an immense promise, fundamentally altering our approach to understanding molecular biology and developing life-saving medicines. It is ushering in an era characterized by unprecedented speed and accuracy, transforming scientific research from a slow, iterative process into a dynamic, AI-accelerated quest for cures.

The continued synergy between artificial intelligence and scientific innovation is not just a trend; it is the future. We are on the cusp of a new paradigm where diseases are understood and treated with unparalleled precision, a future that AlphaFold 3 is helping to build today. The potential to unlock new treatments and improve human health is truly boundless.
For more insights into cutting-edge AI in science, explore our post on **10 Cutting Edge AI Technologies Shaping the Future**.
Understand the broader implications of how **AI is changing the world** and revolutionizing various sectors.
Discover **revolutionary AI medical breakthroughs** that are transforming healthcare.
Explore how **AI is transforming businesses** and driving efficiency.
Learn about **5 Innovative Ways to Use AI Strategies to Boost Productivity**.
Frequently Asked Questions
Q1: What is the primary advancement of AlphaFold 3 compared to previous versions?
AlphaFold 3 can predict the structures of complexes involving proteins, nucleic acids, and small molecules with atomic accuracy, significantly expanding its scope beyond single protein structure prediction. It also models chemical modifications.
Q2: How does AlphaFold 3 impact the drug discovery process?
It accelerates drug discovery by improving target identification, optimizing drug design, predicting potential toxicity, and reducing the need for extensive laboratory experiments, thereby saving time and resources.
Q3: Which companies are collaborating with Isomorphic Labs on AlphaFold 3?
Leading pharmaceutical companies like Novartis and Eli Lilly are collaborating with Isomorphic Labs to integrate AlphaFold 3 into their R&D processes.
Q4: What are some of the challenges facing AI in structural biology?
Challenges include the proprietary nature of some structural data, the need for more diverse datasets, and accurately modeling complex or transient molecular interactions.
Q5: How does AlphaFold 3 achieve its accuracy?
It utilizes advanced deep learning architectures and a diffusion-based modeling technique to construct joint 3D structures of molecular assemblies with high precision.